Clinical Trial Database Lock Checklist
The data manager has to ensure completion of all visits and assessments by all study subjects before initiating database lock. It should be used at the pre-lock meeting to ensure all items have been completed before the database is locked.

All About Clinical Trial Data Management Smartsheet
In order to prepare for an interim or final analysis of a clinical trial the datasets to be analysed must be finalised.
Clinical trial database lock checklist. The process of locking a clinical trial database is an action taken to prevent further changes to the database. On rare occasion even after all queries generated by the clinical and data team have been resolved a statistical programmer a statistician or a medical writer on the team will notice an inconsistency in a data value. This database is available to the clinical trial team for analysis and.
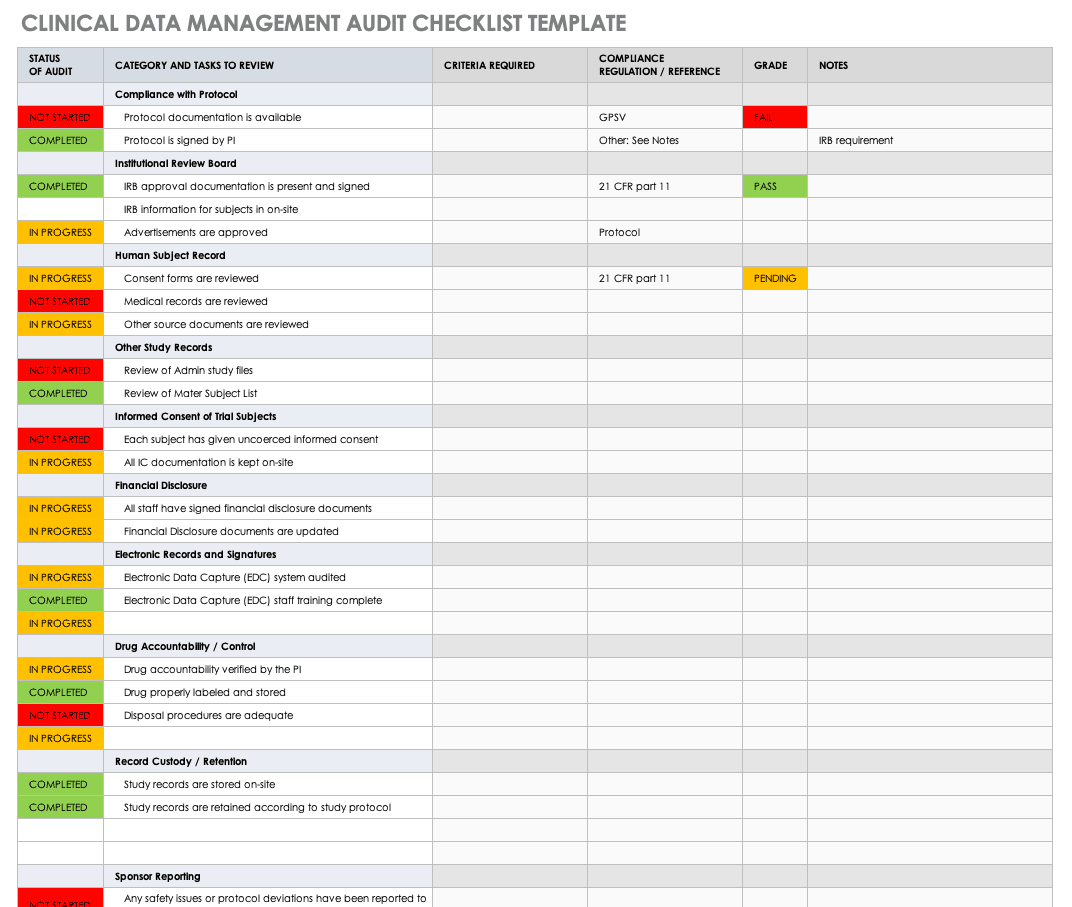
Some of the activities included in database lock checklist are All discrepancies closed DCFs received and updated coding complete SAE Reconciliation process complete etc. Describe the set of closure procedures that will be performed prior to database lock to verify the integrity and completion of the database. Once a study is complete the database manager should lock the database so that no one can change the data.
Other appropriate checks at this stage may include. - Make trial database ready for analysis. 19986265-74 From database build to database lock clinical data management CDM the process of collecting cleaning and managing subject andor trial data in compliance with regulatory standards Krishnankutty B Bellary S Kumar NB Moodahadu LS.
Of course there are exceptions to every rule. Creation Maintenance of Investigator Brochure. Clinical trial data management technology Guide I.
By the QA department of all members involved in the clinical trial process can. Case Report Form Design. To ensure this a pre-lock checklist is used and completion of all activities is confirmed.
Resolve any pending monitoring findingsqueries note. Database lock for a study is done once all data management activities are completed. This checklist will be prepared by CROMS and used by OCTOM in coordination with the Program Official or DIR Clinical Research Staff to determine when all requirements have been met for a site close-out visit.
Re-locking the database should follow the same process for notificationapproval as the initial lock. A database lock must be requested by the SM using a database lock request form based on an agreed template. Data may be entered or corrected on Case Report Forms CRFs until the database has been locked and the study terminated with the IRB Review and ensure all essentialregulatory documents are current complete accurate and filed appropriately and make available to monitor at closeout visit.
Notification of a Serious Breach. ITEM COMMENTS COMPLETE 1 All active clinical research participants have completed their final visit including follow-up visits. Verify all participants are off study at all accruing sites.
Object data management is to achieve high-quality real data. A database is locked after review query resolution and determination that it is ready for analysis. Clinical Trial Data Audits A clinical trial data audit is a review of the information collected in order to ensure the quality accuracy and appropriateness for the stated research requirements per the study protocol.
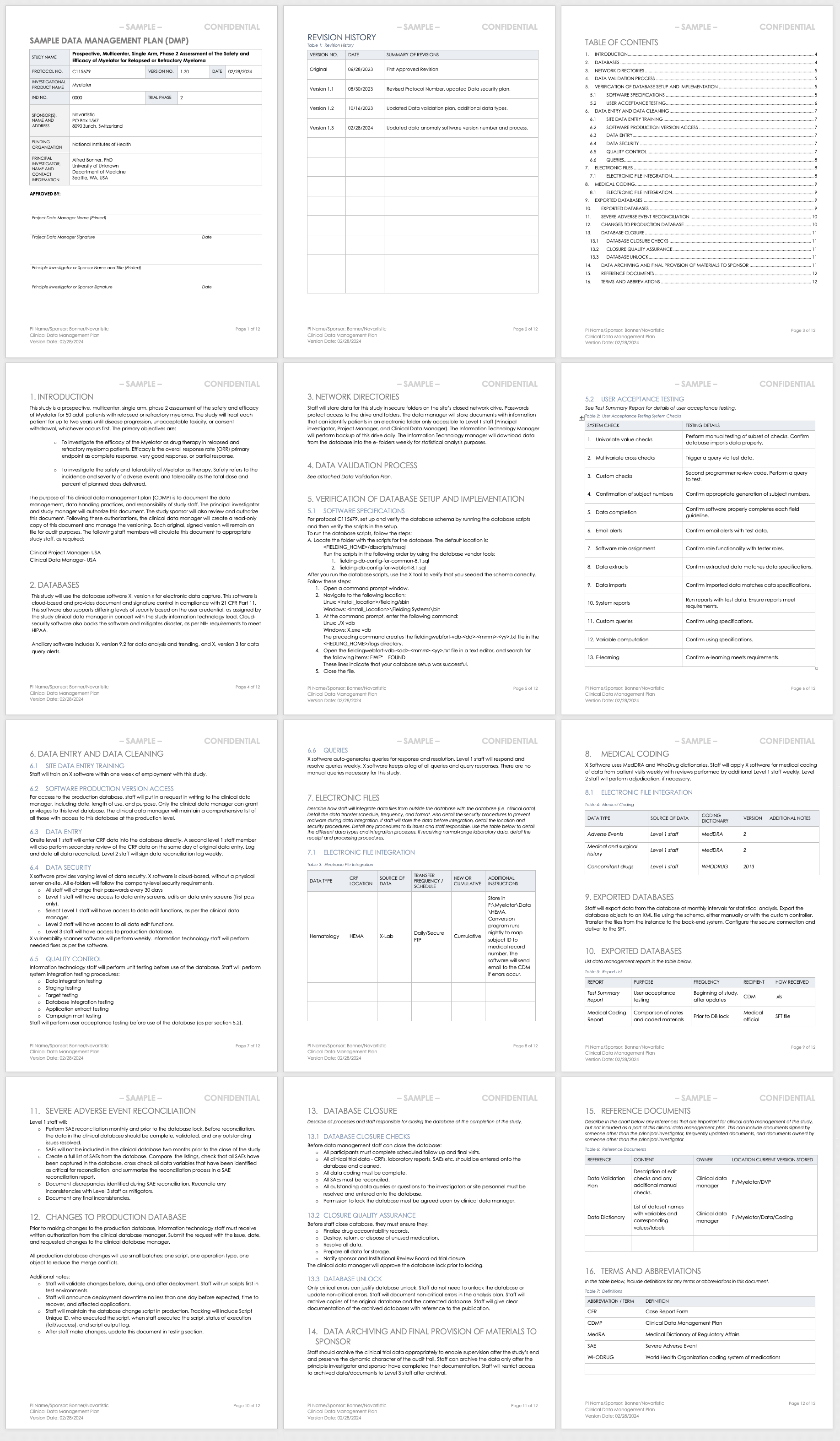
Everests foundation is built on excellence in clinical data management from trial case report form CRF design through to database lock. Obtaining Clinical Trial Insurance KCL Employed Investigators. The data is subjected to stringent scrutiny for validation by database managers who query the site personnel or investigator in case of any discrepancies and prepare a clean database.
Writing a GCP Compliant Clinical Trial Protocol. SOP 825 CDMS VALIDATION The form must be completed signed and submitted to the DBM who will arrange to lock the database at the requested time. Database Lock is achieved after thorough review performed by Data Management Site Monitors Statistician and Clinical Scientist.
Database lock is required - To prevent any further changes to the trial database. Explore 391295 research studies. Overview Clinical Trial Data quality is evaluated on the basis of clinical trial results.
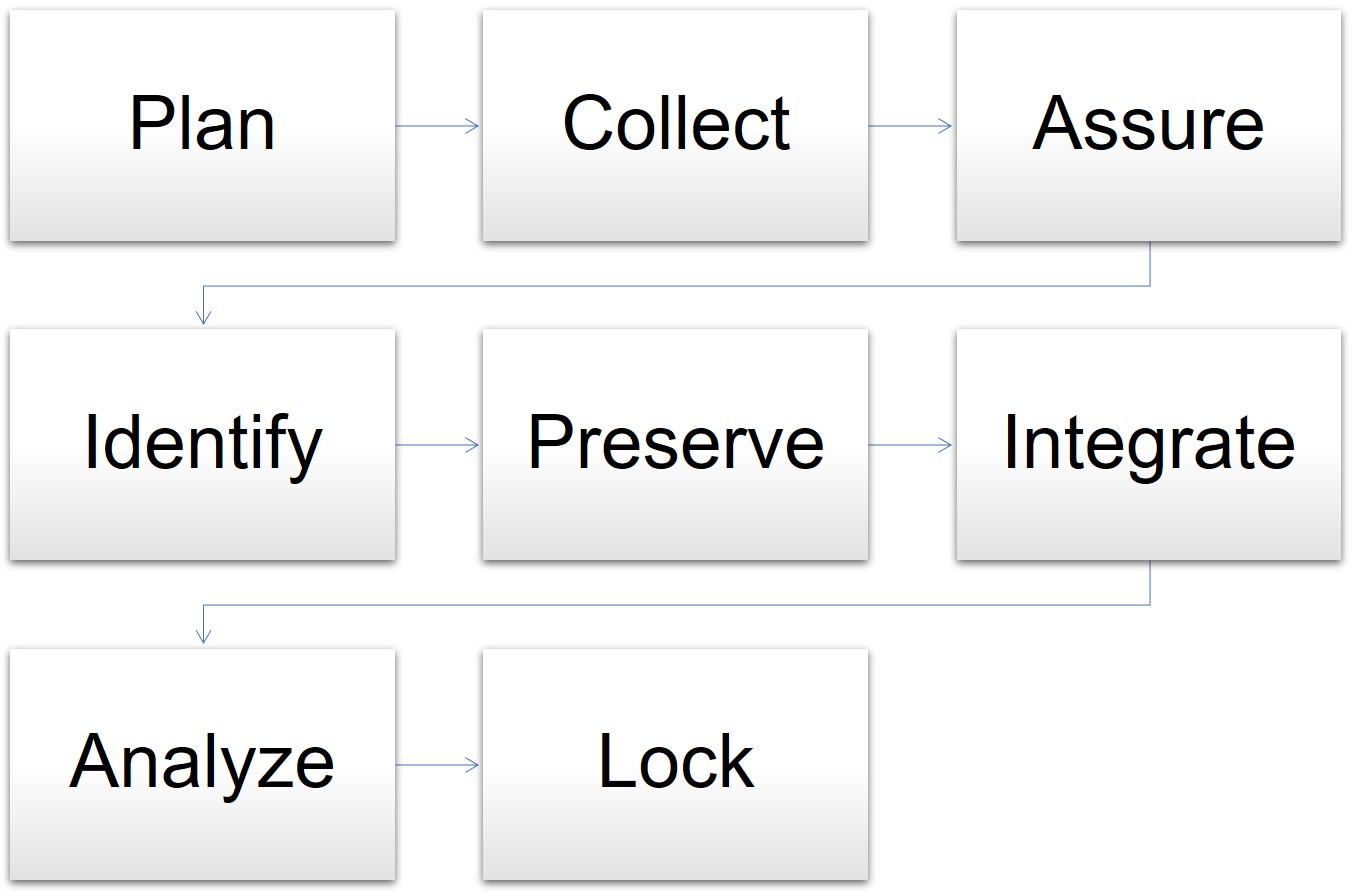
Once hard lock is declared the database is considered final and should never be unlocked. During clinical trials a Clinical Data Management System CDMS is used to store the data generated from various sets of trials. All data management activities should have been completed prior to database lock.
The lock of a database is a very important milestone in a study and once the final database lock has. Clinical data archiving includes planning implementing and maintaining a repository of documents andor electronic records containing clinical information supporting documentation and any interpretations from a clinical trial. On receipt of the Database Lock Request form the DBM will take the.
Once all of the data have been transferred and captured in the database final cleaning reconciliation and verification activities can be. This document lists the items to be checked prior to final lock of a study database. This is done as the database cannot be changed in any manner after locking.
Everest offers both electronic data capture EDC and. Discuss study closeout requirements and study-specific issues with DCP Medical Monitor Scientific Monitor and Nurse Consultant prior to initiating study closeout activities. Management blind review database locking data export and transfer of data and data management documents the archiving.
This includes the database lock checklist which ensures the same. In some cases these may be the same data checks described in above sections repeated until all queries are resolved and the data are determined to be clean. Once the approval for locking is obtained from all stakeholders the database is locked and clean data is extracted for statistical analysis.
Execution excellence has been used by many clinical trial sponsors when describing their experience with Everests clinical data management services. ClinicalTrialsgov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. Clinical Database is a databank where data from different clinical trial sites is entered by site professionals or investigators via any Clinical Data Management System CDMS.
The International Conference on Harmonization Good Clinical Practice guideline.

Pdf Electronic Data Capture In Clinical Trials Interface Design And Evaluation And System Validation Semantic Scholar
All About Clinical Trial Data Management Smartsheet
All About Clinical Trial Data Management Smartsheet
Posting Komentar untuk "Clinical Trial Database Lock Checklist"