Arisg Database Guide
There are two options for submitting ICSRs electronically. The PSL web service uses this User Context to perform updates in the Argus Safety Database.
Compare best arisg safety database training courses online from top Platforms Universities.
Arisg database guide. Get access to a demo Oracle Argus Safety software here. It is the main repository for collected safety information for the companys products it facilitates the reporting of individual and aggregate safety data to authorities and third parties and provides a key source of information for ongoing detection of. On Full Data Entry Study Screen the data gets reflected while selecting the protocol from protocol lookup.
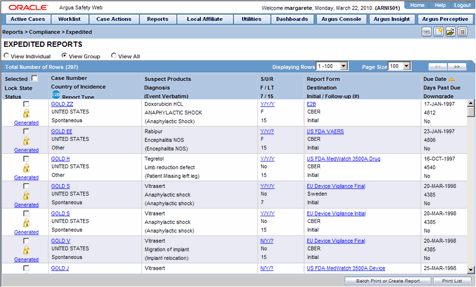
Viewing New Cases in the Worklist. Train yourself in Oracle Argus Safety by clicking on each user guide link here and making sure you understand the steps. Viewing your Pending Action Items.
HAROM SOLUTIONS database management it doesnt involve case processing 4. The existing AER number selected from the ARISg database to reclassify the received case. Software used in Pharmacovigilance Oracle Argus Safety.
Synonym lists can be used to help auto-code verbatim terms and reduce manual eforts. In this document file names and user -defined content are formatted and identified as follows. This is the TNS of the Argus database.
Pharmacovigilance Pharmakon -drug Vigilare to keep watch Pharmacovigilance PV Drug Safety. A Global Pharmacovigilance Safety Database sits at the heart of the vigilance system for medicinal products and devices. Aris Globals LifeSphere Safety otherwise known popularly as ARISg is another market-leading pharmacovigilance database with a long and well-established heritage in the industry.
Hi everyone Here is the list of Pharmacovigilance PV companies in India. PPD was able to easily extract the data from the ARISg database to identify potential cases and use the system to complete the remaining requested activities. Before selecting the existing AER number from the ARISg database this field displays the current AER number of the case.
Interactive Stories Scavenger Hunts Tours Data Collection Activities etc. Whats New in ARISg 736. Arisg safety database training.
The Admin Protocol Maintenance screen has been enhanced by introducing a new check box EU CT Regulations 2019 to determine if the protocol is included in the new EU CT Regulations or not. Find Top Paid Free online arisg safety database training courses certifications trainings programs specialization at Naukri Learning. Finding a Case Assigned to you.
Compare best arisg safety database training courses online from top Platforms Universities. FAERS VAERS online datasets for safety monitoring and online filing of AEs. ArisGlobal is transforming the way todays most successful Life Sciences companies develop breakthroughs and bring new products to market.
Database-to-database transmission E2B The Safety Reporting Portal SRP. Oracle Argus Safety Offers Better Data Insights and Faster decision making. This is the user name of a AG Service user.
- ARIS exists as an experimental platform to expand what is possible. ELITE LIFE SCIENCES 3. PV SOLUTIONS database management it doesnt involve case processing 3.
Page 18 - Find Top Paid Free online arisg safety database training courses certifications trainings programs specialization at Naukri Learning. ARIS is an open-source easy-to-use platform for creating and playing augmented reality experiences on iOS devices. It allows for all pharmacovigilance processes from case entry to automatic generation of submission ready adverse event AE reports including CIOMS 1 MedWatch 3500A and many more.
Pharmacovigilance It is the pharmacological science relating to the collection detection assessment monitoring and prevention of adverse reaction with Pharmaceutical products. Enter the AER number of the existing case in ARISg to which the selected case must be reclassified. Generate new encrypted string as mentioned in the Generating Encrypted String from Clear Text on Configured User Cryptography Key section.
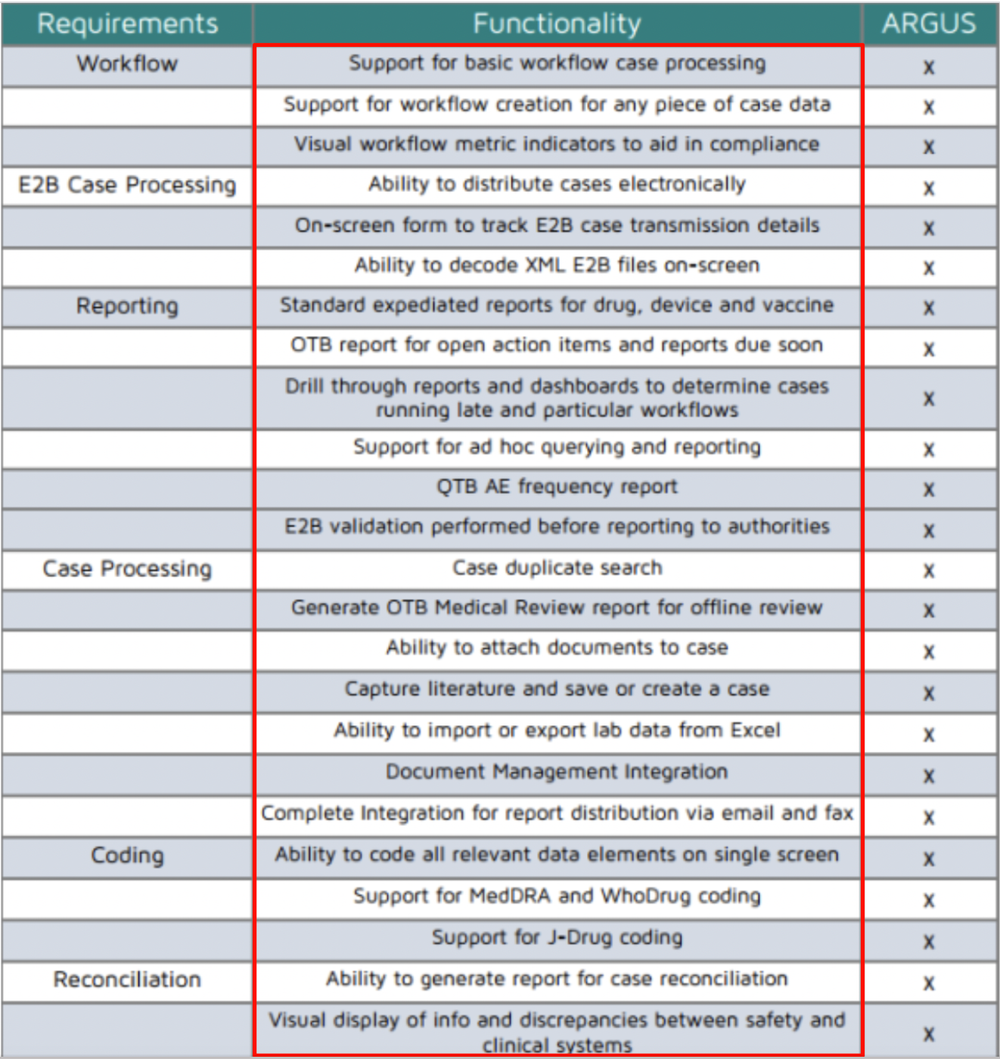
Oracle Argus Safety Certification is a 2 day 6-module program that covers every tab and task needed to manage drug safety data when using the Oracle Argus Safety Training application. We can customize fields and configure workflows in the database based on client requirements and expedite user access authorizations in a cost effective manner which saves. This safety database is web based and essentially designed specifically for pharmacovigilance services activity to gather assess and report adverse event information to the appropriate worldwide regulatory.
Our end-to-end drug development technology platform LifeSphere integrates our proprietary Nava cognitive computing engine to automate all core functions of the drug development lifecycle. Answer 1 of 8. Pharmacovigilance PV software also Safety databases comprises drug safety management software solution that enables to create classify review submit and maintain pharmacovigilance data and adverse event reports also known as ICSRs.
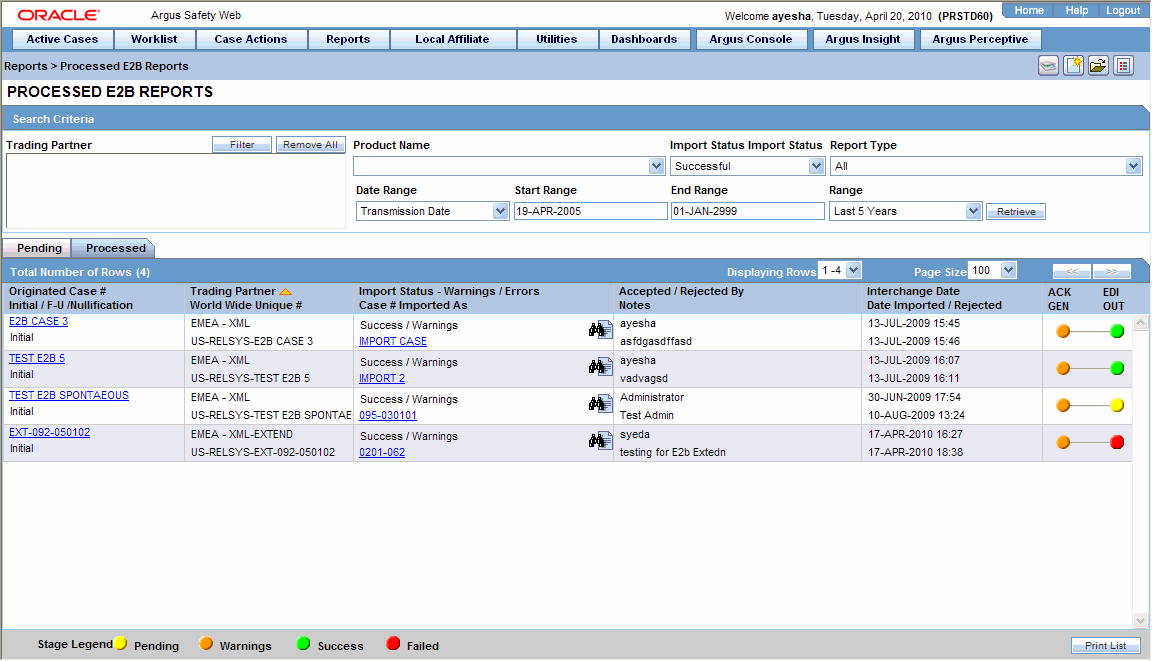
ARISg provides all the functionality required to manage adverse event reporting and adverse reaction requirements of different regulatory authorities around the world. Attaching Additional Information to your Case. In case MedDRA and WHO Drug codes were not included in the incoming E2B report coding will be the next step in the process.
Also the new field. Viewing your Worklist Action Items. Oracle Argus Safety Ensures Global Regulatory compliance.
Oracle Argus is a market leading pharmacovigilance database. Oracle Argus Safety I. This manual describes the settings and features as they were at the time of print.
PAREXEL Acquired Quantum solutions 2. PPDs pharmacovigilance department was able to generate 260 SIE narratives perform extensive site follow-up and provide 110 death narratives with source documentation to the adjudication panel. It provides comprehensive suite of functionality and tools as well as additional modules and interfaces covering other aspects of pharmacovigilance as well as regulatory medical affairs and clinical.
Populated automatically and the traditional manual entry of data is almost entirely avoided. Viewing your Coding Action Items. 22 Using Oracle or Microsoft SQL Server instead of the Standard Database System.
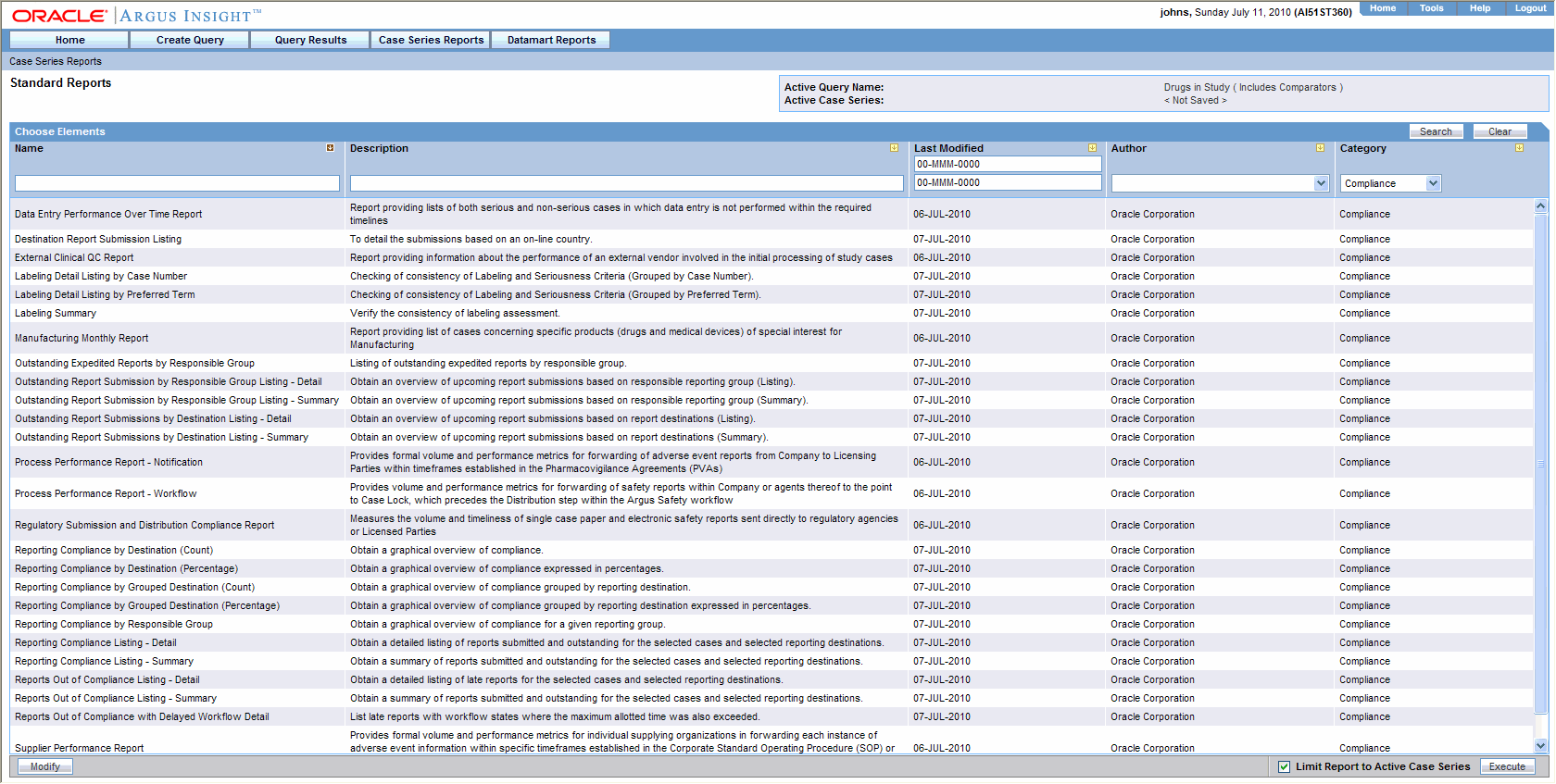
See a skim-through of our Argus Safety Database training and free guidesresources below. This PV software is used by pharma and biotech companies contract research organizations CROs. Performing a Medical Review.
By implementing this Multi-Tenant Architecture of ARGUS database iSafety is compliant to ICHHL7 guidelines following regulatory standards with periodic updates WHO DD MedDRA coding. This guide to some key features of the ARISgTM safety database can help you understand what it does and why it features so heavily in European pharmacovigilance work. Designed with deep expertise.
Argus Software Training P Clinical Research Blog Certified Clinical Research Professionals Society Clinical Research Certification

Aris Global Product Overview Agxchange Irt And Ost Inbound Receipt And Triage Outbound Submissions Tracking Pdf Free Download
Posting Komentar untuk "Arisg Database Guide"