Database Lock Clinical Trial
At this stage the data is declared final terminology varies but common descriptions are Database Lock and Database Freeze and the clinical data manager transfers data for statistical analysis. Database lock must be documented with proof that edit access was removed at a definitive point in time.

Competency Framework For The Clinical Research Professional Clinical Research Research Clinic
Important in randomized blinded.

Database lock clinical trial. This is mainly because clinical trial database design is a slow inflexible process which has remained largely unchanged over the past 20 years. 4 Prevents unauthorized unintentional changes. Of the 346 patients enrolled ineligible patients and patients who did not receive bevacizumab were excluded and the remaining 333 patients were classified as treated patients.
A database is locked after review query resolution and determination that it is ready for analysis. Database lock may occurwhen all patients complete Week104or discontinue study treatment prior to the end of the randomized double-blind withdrawal period. During a clinical trial a lot of effort goes into data management before final database is lock or the hard lock happens.
LPLV and database lock. Database lock A clinical trial term of art for an action taken to prevent further changes to the trial database. Clinical trials also are used to study interventions that do not involve regulated medical products such as surgical techniques behavioral interventions means of improving disease management practice or changes to a health care system Califf 2013.
The whole story begins with finalization of study protocol. What is database lock in clinical trial. Clinical Trial Database Lock Date means the date when the clinical research database relating to the Clinical Trial is locked after the Clinical Trial Results have been cleaned but excluding any Long Term Survival Data in accordance with the Charitys Standard Operating Procedures.
Guidelines for Hard-Locking a Clinical Research Database May 27 2010 Clinical Research Education For every clinical research study there is a beginning several interim milestones and a definite endpoint. Lock the data and unlock upon request only. A database is locked after review query resolution and determination that it.
In big oncology or cardiovascular trials it may be difficult to have a database that is completely clean. In order to prepare for an interim or final analysis of a clinical trial the datasets to be analysed must be finalised. At this time unblinding will occurand the primary analysis will be performed.
Database Lock means with respect to any Clinical Trial the date on which the databases containing the applicable clinical trial data is determined to be complete and locked in order to permit the analysis of the primary and secondary endpoints of such Clinical Trial. Certainly you could have a 100 SDV in a NIS and perform the data cleaning for your interim analysis as you would for a clinical trial ie. A trial data manager plays a key role in achieving successful database lock by acting as liaison between statisticians clinical and technical personnel and ensuring that every step related to data collection and verification was followed properly.
Review and finalization of study documents. The database lock is one of the final steps taken during a clinical trial before submitting the information to the FDA. Follow-up was terminated in February 2017 and data were locked in October 2017.
As a clinical trial is designed to answer the research question the CDM process is designed to deliver an error-free valid and statistically sound database. At the completion of the clinical trial the clinical data manager ensures that all data expected to be captured has been accounted for and that all data management activities are complete. Therefore any clinical trial must have a well defined process for closing its database and clear change-control procedures for re-opening the database if necessary.
Database lockunlock - This is a controlled procedure to freeze data to prevent writeedit access to users of the system. For the clinical research database the initial milestone is normally finalization of. To meet this objective the CDM process starts early even before the finalization of the study protocol.
However this is an unrealistic scenario in the vast majority of NIS in a study with thousands of patients it is likely that dealing with data unlock-requests would be more than a full-time job. Also all external data such as the safety lab database or other vendor databases should have been cleaned and reconciled. Quotes from a clinical trial report JGOG3022 trial.
Prior to the final database lock a soft lock is declared. Database lock is done once all data entered has been cleaned with no outstanding queries or discrepancies. The process of locking a clinical trial database is an action taken to prevent further changes to the database.
What is a database lock. Any comments from the team on dry-run outputs are addressed and applied to the database for a final round of cleaning. Explain how approval for final database lock will be sought and documented and how the lock will be achieved at the database level.
3 2 1 PI signs all data from the beginning until the end which is then entered in eCRFs. Consider the nature of the study eg minimal risk study clinical trial requiring an IND and indicate. It is done after a final quality check and data validation but before the data is extracted and formatted for submission.
The team then prepares for further analysis interpretation and reporting of findings in a clinical study report CSR. 4 Thus as the committee examined what clinical trial data should be shared and when it was useful to consider clinical trials in these two broad categories. At the time of database lock instead of checking all outstanding queries it could be better to focus on specific data and base the decision of resolving or not the.
A final database lock will occur after the Post-Treatment Follow-Up Period Period 4 is. The clinical trial team reviews the dry run outputs which provide a glimpse of the final outputs.
Clinical Data Management Processes After Data Collection Clinskill

Acorn Cro Providing Value Driven Customer Centric Oncology Focused Solutions Oncology Study Design Solutions
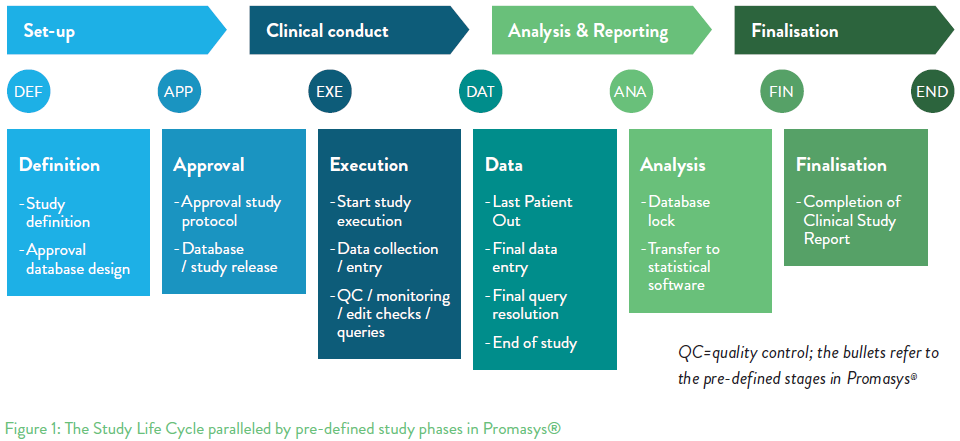
Posting Komentar untuk "Database Lock Clinical Trial"